|
Hip Implant Surgery
and Potential Lawsuits
Hip replacement surgery, also called total
hip arthroplasty, involves removing a diseased or broken hip
joint and replacing it with an artificial joint, called a
prosthesis. Hip prostheses commonly consist of a ball component,
made of metal or ceramic, and a socket, which has an insert or
liner made of plastic, ceramic or metal. The implants used in
hip replacement are or should be biocompatible (meaning they're
designed to be accepted by your body) and made to resist
corrosion, degradation and wear.
As a total hip joint replacement replaces the
ends of both bones in a damaged hip joint to create new joint
surfaces and a total hip replacement surgery replaces the upper
end of the thighbone (femur) with a metal ball and resurfaces
the hip socket in the pelvic bone with a metal shell and plastic
liner, it is essential that the hip implants are biocompatible
and are correctly made to resist corrosion, degradation, and
wear as well as to work well without rubbing.
There are currently four device options for total hip
replacement in the United States. These are:
- Metal-on-Polyethylene: The
ball is made of metal and the socket is made of plastic
(polyethylene) or has a plastic lining.
- Ceramic-on-Polyethylene: The
ball is made of ceramic and the socket is made of plastic
(polyethylene) or has a plastic lining.
- Metal-on-Metal: The ball and
socket are both made of metal.
- Ceramic-on-Ceramic: The ball
is made of ceramic and the socket has a ceramic lining.
The FDA's Concerns
about Metal-on-Metal Hip Implant Systems
All artificial hip replacement systems have risks related to
implant or material wear. Metal-on-metal hip (MoM) replacement
systems have unique risks in addition to the general risks of
all hip implant systems.
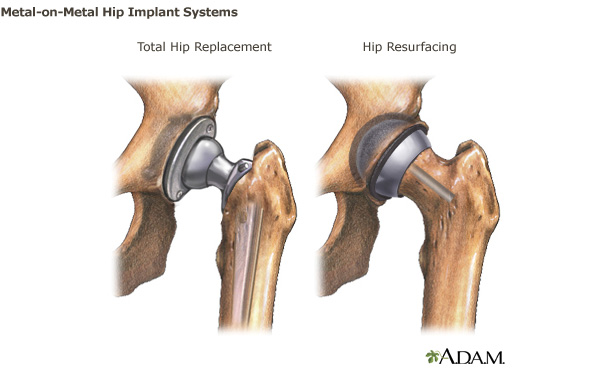
Because the metal ball and the metal cup slide against each
other during walking or running, some tiny metal particles may
wear off of the device and enter into the space around the
implant. Some of the metal ions from the metal implant or from
the metal particles may even get into the bloodstream.
Orthopaedic surgeons take several precautions before and
during the implantation surgery to try to optimize the way in
which the ball and socket rub against each other so that fewer
wear particles are produced. However, there is no way to fully
avoid the production of metal particles.
Different people will react to these metal particles in
different ways. At this time, it is not possible to know who
will experience a reaction, what type of reaction they might
have, when the reaction will occur, or how severe the reaction
will be. However, it is known that over time, the metal
particles around some implants can cause damage to bone and/or
tissue surrounding the implant and joint. This is sometimes
referred to as an “adverse local tissue reaction (ALTR)” or an
“adverse reaction to metal debris (ARMD).” Such a reaction may
cause the implant to become loose or cause pain. Ultimately this
can require a revision surgery where the old device is removed
and replaced with another one.
In addition to these reactions to metal near the joint and
implant, there are some case reports in the literature of a
small number of patients in which high levels of metal ions in
the bloodstream may have caused other types of symptoms or
illnesses elsewhere in the body, including effects on the heart,
nervous system, and thyroid gland.
Patients who have MoM hip implants should be aware of
potential symptoms which may occur after surgery and indicate
that their device is not functioning properly. Common symptoms
may include:
- Pain in the groin, hip or leg
- Swelling at or near the hip
joint
- A limp or change in walking
ability
There are many reasons why any of these symptoms may arise or
worsen after surgery and it does not necessarily mean that a
reaction to the metal particles from the device is taking place.
A thorough evaluation by an orthopaedic surgeon is needed to
determine the actual cause. Besides a physical exam of the hip,
the orthopaedic surgeon may consider several tests to evaluate
these symptoms including:
- Special imaging tests
- Using a needle to remove fluid
from around the joint (joint aspiration)
- Blood tests, including
checking levels of metal ions in the blood
Patients who receive a MoM hip implant should also pay close
attention to changes in their general health or
new symptoms
outside their hip including symptoms related to their:
- Heart (chest pain, shortness
of breath)
- Nerves (numbness, weakness,
change in vision or hearing)
- Thyroid (fatigue, feeling
cold, weight gain)
- Kidney (change in urination
habits)
Other Defective hip Implants
Failed hip implant surgery including
defective hip implants have been becoming more and more
common. Some hip implant medical devices that have been the
subject of failed hip surgery lawsuits, recalls, or FDA actions
include the Zimmer Durom Cup, the Stryker Corp. Trident
Hemispherical and Trident PSL cups, DePuy ASR Hip Resurfacing
Systems, and Depuy ASR XL Acetabular Systems.
Through failed hip replacement
lawsuits many patients are alleging that defective hip implants
including defective Zimmer Cups, Trident Hemispherical and PSL
Cups, or DePuy Orthopaedics Inc. ASR Hip Resurfacing
System and the ASR XL Acetabular Systems
have caused them pain, suffering, impairment, and the need for
additional hip replacement surgeries.
The Zimmer Durom Cup has been implanted into
over 12,000 people and is primarily used in Total Hip
Replacement surgeries. In July 2008 sales of the Zimmer Durom
Cup were suspended by Zimmer, after reports that the cup was
defective and failed to bond in many patients. This failure to
bond caused these patients to have to undergo painful revision
hip surgeries.
The Food and Drug Administration (FDA), has
warned the Stryker Corporation about the Trident Hemispherical
and Trident PSL cups that have "failed to function" as well as
about hip implant components with "poor fixation" These cup or
failed implant have also required painful revision hip
surgeries. The latest warning letter released by the FDA
indicated that the Stryker Corporation received multiple
complaints from 2005 through 2007 involving devices that failed
to work and hip implant components that had bad fixation. Some
hip implant failures have required that hip implant patients
undergo a follow up surgery to fix the problem with the first
hip implant surgery. These problems in addition to the failure
to function and poor fixation include the issue of terrible
squeaking noises coming from the hip implant.
The FDA has alsowarned manufacturers of hip
implants that the recurrence of squeaking noises of hip implants
with ceramic bearing components have resulted in revision
surgeries due to implant failures (fractures, pain, wear
particles, and fragments) and many patients have and are
expected to experience problems including Hip Pain and may
require additional hip implant surgery to repair the defective
hip implant devices.
In August, 2010, DePuy Orthopaedics Inc.
announced that it would be recalling two hip replacement
products, the ASR Hip Resurfacing System and the ASR XL
Acetabular System, after receiving new data which indicated that
more patients than expected experienced pain and other symptoms
which required a revision surgery following the initial hip
replacement procedure. The patients with DePuy ASR and ASR XL
metal-on-metal hip implants have experienced loosening and
dislocation of the device resulting in the need for early
revision surgery and/or the release of metal debris causing
muscle and soft tissue damage. For more information on
Depuy hip replacement failures
click here
Patients with Serious Hip Injuries when
Seeking Pain Relief can sometimes Fall Victim to Defective
Products, Negligent Health Care, and "For Profit" Medical
Professionals
Unfortunately, there are medical
clinics, medical implant sales people, and doctors that are more
interested in profit and their bottom line than what is best for
a patient. Some of these medical professionals carelessly cause
painful and difficult conditions to become much worse by pushing
defective medical devices or pushing surgeries when other less
drastic medical options are available.
As such, it is usually best to
get a second or third opinion prior to agreeing to have a hip
surgery operation as well as to make sure that you trust your
medical providers and are sure that they are working in your
best interest.
Defective
Hip Implant Devices Lead to Suffering Pain, Impairment, and Mental Anguish as well
as having to have Second Hip Replacement Surgery to Repair the
Problem
Revision Surgery: one of the most serious and
prolonged injuries resulting from DePuy defective hip
replacement systems, is the need for surgical extraction of the
faulty ASR Hip Implant Device and often replacement with another
defect-free Hip Replacement System of proper design by other
orthopedic device manufacturer. The Hip replacement by its very
nature of necessity is often surgically performed in middle-aged
and elderly population. And recovery from the second hip
replacement is often more difficult than after the first. A
second surgery of this nature could and often does increase the
risk of complications, severe infections, prolonged recovery
time, and death. In some patients the infection resulting from
defective ASR system, is so advanced and systemic that a
revision surgery is not possible. In this group of patients the
only option is a surgical extraction of defected device without
any corrective hip replacement to restore the lost mobility
function patients experienced in first place.
After a person has gone through an extensive
hip surgery including the lengthy recovery and process, it can
be very upsetting to learn that the manufacturer incorrectly
made the implant so that it will have to removed and the person
will have to undergo the hip replacement surgery again requiring
more recovery, missed time from work, and missed time from
family and normal functioning.
Regardless, as
to whether the failed hip replacement surgery is caused
by a defective Zimmer Cup, defective Trident Hemispherical Cup,
defective Trident PSL Cup, defective DePuy Orthopaedics
Inc. ASR Hip Resurfacing System, defective DePuy ASR XL
Acetabular System, and/or medical
negligence, it is important to understand the cause of a failed
hip replacement surgery.
If you or a
family member have been the victim of a failed hip replacement
surgery including a defective hip replacement cup implant or hip
replacement system, please feel free to use the
submit an inquiry or
click here to send an e-mail with any questions about a failed hip replacement surgery and
defective hip implant lawsuit.
|

